-
Athira Pharma Announces Topline Results from ACT-AD Phase 2 Proof of Concept Study of Fosgonimeton in Mild-to-Moderate Alzheimer’s Disease
来源: Nasdaq GlobeNewswire / 22 6月 2022 06:00:01 America/Chicago
Primary endpoint of change in biomarker ERP P300 latency was not statistically significant for the full study population as combination of fosgonimeton and standard-of-care (AChEIs) given together showed potential diminished effect of fosgonimeton
A pre-specified subgroup analysis of patients on fosgonimeton monotherapy suggests improvement in both ERP P300 latency and ADAS-Cog11 at week 26 compared to placebo indicating pharmacological activity
Fosgonimeton had a favorable safety profile over 26 weeks and ACT-AD provides important learnings for ongoing LIFT-AD study
Athira to host live webcast today at 8:30 am Eastern time
BOTHELL, Wash., June 22, 2022 (GLOBE NEWSWIRE) -- Athira Pharma, Inc. (NASDAQ: ATHA), a late clinical-stage biopharmaceutical company focused on developing small molecules to restore neuronal health and slow neurodegeneration, today announced topline results from its exploratory ACT-AD Phase 2 study of fosgonimeton (ATH-1017) in patients with mild-to-moderate Alzheimer’s disease (AD). Fosgonimeton is a small molecule designed to enhance the activity of Hepatocyte Growth Factor (HGF) and its receptor, MET, which are expressed in the central nervous system to promote brain health and function.
“Following compelling ERP P300 latency biomarker data from a small Phase 1b trial over eight days in Alzheimer’s patients on fosgonimeton monotherapy, this Phase 2 trial provides valuable insights into the nature of this novel intervention over 26 weeks. ACT-AD was designed as a learning study to further investigate the ERP P300 biomarker signal over 6 months, assess safety in a patient population more representative of the real world, by allowing the use of add-on standard-of-care acetylcholinesterase inhibitors (AChEIs, e.g., donepezil), and explore fosgonimeton’s effect on psychometric outcomes, including ADAS-Cog11, to inform the ongoing Phase 3 LIFT-AD study. To that end, this study achieved its goal,” said Hans Moebius, M.D., Ph.D., Chief Medical Officer of Athira.
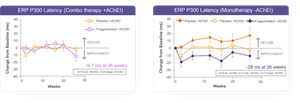
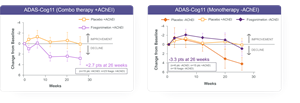
“The study was intended to show differences on the biomarker ERP P300 latency. This primary endpoint was not met by protocoled analysis, however a pre-specified subgroup analysis indicated a potential diminished effect of fosgonimeton when given in combination with AChEIs. A subsequent post hoc analysis of the data from patients on fosgonimeton monotherapy showed a meaningful improvement in both ERP P300 latency (-28 milliseconds) and cognitive performance (ADAS-Cog11: -3.3 points) compared to placebo at 26 weeks.
“These data points are very encouraging as they indicate the expected pharmacological activity of fosgonimeton by parallel improvement on ERP P300 latency and ADAS-Cog11 and show a favorable safety profile over six months. This is the first time monotherapy fosgonimeton has shown an effect on ADAS-Cog11, suggesting a potential cognitive benefit. We will use these insights for a rational optimization of the ongoing LIFT-AD trial. We plan to seek advice from our scientific advisors, investigators, and ultimately regulators on how to expeditiously analyze and potentially adapt the LIFT-AD study,” added Dr. Moebius.
“The data from the fosgonimeton monotherapy analysis are encouraging and show biologic activity that may support the potential role of the HGF/MET pathway in neurodegenerative diseases,” said Marwan Sabbagh, M.D., FAAN, professor of neurology at Barrow Neurological Institute, Phoenix, AZ. “ACT-AD adds to the body of literature suggesting ERP P300 latency as an important biomarker for cognitive status.”
ACT-AD Study Design and Results
ACT-AD was an exploratory, randomized, double-blind, placebo-controlled, parallel-group 26-week trial evaluating fosgonimeton compared to placebo in patients with mild-to-moderate Alzheimer’s disease. The study enrolled 77 patients in the United States and Australia (age 55 to 85 years, Mini-Mental State Exam (MMSE) score of 14-24 and Clinical Dementia Rating (CDR) scale global score of 1 or 2). Patients were allowed to continue standard-of-care therapy (AChEIs), with 60 percent remaining on stable doses of AChEIs and 40 percent not receiving AChEIs during the study. Patients were randomized 1:1:1 to receive placebo or fosgonimeton at either 40 mg/d or 70 mg/d. The primary endpoint for ACT-AD was Event-Related-Potential (ERP) P300 Latency, a functional measure of working memory processing speed. Secondary endpoints included ADAS-Cog11, a measure of cognition; ADCS-CGIC, a measure of global clinical change; and ADCS-ADL23, a measure of functional change. Safety data were evaluated throughout. The study was only powered to show statistical significance for change in ERP P300 latency.
The ACT-AD study did not meet the primary endpoint of a statistically significant change in ERP P300 Latency for the modified intent to treat (mITT) population by a mixed model repeated measures (MMRM) analysis (-6.02 milliseconds) when compared with placebo at 26 weeks in a pooled analysis of the 40 mg and 70 mg dose groups. Secondary endpoints, including ADAS-Cog11, ADCS-CGIC, and ADCS-ADL23, were not significant in treated subjects compared with placebo at 26 weeks. A pre-specified subgroup analysis identified a potential diminished effect of the combination of standard-of-care (AChEIs) and fosgonimeton. Other subgroup analyses, to-date, including dose, disease severity and APOE genotype, did not show differences between groups.
A post hoc analysis, based on the mITT population on fosgonimeton monotherapy, showed a potentially beneficial change in ERP P300 compared to placebo at 26 weeks (-28 milliseconds) as well as cognitive improvement as measured by ADAS-Cog11 (-3.3 points) compared with placebo at 26 weeks.
ACT-AD ERP P300 Latency post hoc analysis: mITT population, Wilcoxon analysis
ACT-AD ADAS-Cog11 post hoc analysis: mITT population, Wilcoxon analysis
Fosgonimeton was generally well tolerated, with a favorable safety profile. There were no treatment related Serious Adverse Events or deaths observed in the study. Participants treated with fosgonimeton at 40mg or 70mg for 26 weeks showed a higher incidence of treatment emergent adverse events compared to placebo. The most frequent adverse event was injection site reaction, sometimes associated with transient and asymptomatic increases in absolute Eosinophil count. The study had a 14 percent early termination rate.
Full analysis results are scheduled to be presented at the Alzheimer’s Association International Conference (AAIC) taking place July 31 – August 4, 2022.
“As planned, the ACT-AD study results have provided us with important insights that we will use to inform our ongoing LIFT-AD study, which is enrolling mild-to-moderate AD patients. We are encouraged by these data as they show more than just biologic activity; although a small sample size, they suggest a potentially beneficial treatment effect as a monotherapy that in the ACT-AD study was similar to standard-of-care with a favorable safety profile,” said Mark Litton, Ph.D., President and Chief Executive Officer of Athira.
“We are in the fortuitous situation that we have a much larger trial ongoing with more than 200 patients completing at least 20 weeks of treatment providing us with an opportunity to obtain more insights in an expedited manner. Our strong cash position allows us to continue to progress fosgonimeton development.
“In addition to the biomarker results from both the Phase 1b and this study, these data are the first to show fosgonimeton’s potential effect on a key measure of cognitive improvement in Alzheimer’s disease patients by positive modulation of the HGF/MET receptor by fosgonimeton as a monotherapy. We continue to enroll in the open-label extension study that was recently extended to 18 months. We are grateful to the clinicians and patients, along with their families and caregivers, who participated in this trial and continue to support the scientific community in our endeavors to bring new options to patients in need,” concluded Dr. Litton.
Additional information on the ACT-AD study can be found at: NCT04491006. The ACT-AD trial is supported by a grant from the National Institute on Aging of the National Institutes of Health under Award Number R01AG06268. The information presented in this press release is solely the responsibility of Athira and does not necessarily represent the official views of the National Institutes of Health.
About the Phase 3 LIFT-AD Clinical Study
LIFT-AD is a randomized, double-blind, placebo-controlled, parallel-group Phase 3 study of fosgonimeton for patients with mild-to-moderate Alzheimer’s disease. The study will enroll approximately 420 patients in the United States, with enrollment ongoing. Patients are randomized across two dose groups and one placebo group on a 1:1:1 basis to receive a subcutaneous injection of fosgonimeton or placebo once daily over a treatment course of 26 weeks. The primary endpoint for LIFT-AD will be measured by the Global Statistical Test, which is a mathematical algorithm that combines the scores from cognition (Alzheimer's Disease Assessment Scale-Cognitive Subscale [ADAS-Cog11]), and either global impression of change (Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change [ADCS-CGIC]), or function (Alzheimer’s Disease Cooperative Study-Activities of Daily Living [ADCS-ADL23]). Additional information on the study can be found at: NCT04488419.
About Fosgonimeton (ATH-1017)
Fosgonimeton (ATH-1017) is a small molecule designed to enhance the activity of hepatocyte growth factor (HGF) and its receptor, MET, to impact neurodegeneration and regenerate brain tissue. The function of the HGF/MET receptor system may be impaired in the brain under conditions of neurodegeneration. In addition to Alzheimer’s disease, fosgonimeton has the potential to address the broader dementia population, including Parkinson’s disease dementia and Dementia with Lewy bodies, as the mode of action focuses on network recovery and synaptic signal transmission in the brain.
About Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that currently affects an estimated 6.5 million Americans aged 65 and older, according to the Alzheimer’s Association. AD has multifactorial and complex pathologies that involve the nervous system, vasculature, immune system and neurotransmitters. One early event of AD progression is the loss of 25-36% of synapses, which impacts several brain regions, including the hippocampus and frontal cortex, regions important for learning and memory. In AD, the expression of neuronal MET is reduced by 75% in the hippocampus and 25% in the frontal cortex, suggesting that dysregulation of HGF/MET could be implicated in AD and other brain pathologies.
Live Webcast
Athira will host a live webcast to discuss the ACT-AD results in greater detail at 8:30 a.m. ET today, Wednesday, June 22, 2022. To access the live webcast, please visit the "Events and Presentations" page within the Investors section of the Athira website https://investors.athira.com/news-and-events/events-and-presentations. An archived replay will also be available on the website for at least 90 days following the event.
About Athira Pharma, Inc.
Athira Pharma, Inc., headquartered in the Seattle area, is a late clinical-stage biopharmaceutical company focused on developing small molecules to restore neuronal health and slow neurodegeneration. Athira aims to provide rapid cognitive improvement and alter the course of neurological diseases with its novel mechanism of action. Athira is currently advancing its lead candidate, fosgonimeton, a novel small molecule for Alzheimer’s, Parkinson’s disease dementia and Dementia with Lewy bodies. For more information, visit www.athira.com. You can also follow Athira on Facebook, LinkedIn and @athirapharma on Twitter and Instagram.
Forward-Looking Statements
This release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are not based on historical fact and include statements regarding fosgonimeton as a potential treatment for Alzheimer’s disease, Parkinson’s disease dementia and Dementia with Lewy bodies, and other dementias; Athira’s platform technology and potential therapies; future development plans; clinical and regulatory objectives and the timing thereof; expectations regarding the potential efficacy and commercial potential of Athira’s product candidates; the anticipated reporting of data; the potential learnings from the ACT-AD trial and their ability to inform and improve future clinical development plans; and Athira’s ability to advance its product candidates into later stages of development. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “plan,” “believe,” “intend,” “pursue,” “continue,” and other similar expressions, among others. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, the preliminary data for Athira’s fosgonimeton product candidate from the Phase 1a/b and Phase 2 ACT-AD trials will not continue or persist in current or planned clinical trials; cessation or delay of any of the ongoing clinical trials and/or Athira’s development of fosgonimeton and other product candidates may occur; the impact of the COVID-19 pandemic on Athira’s business, research and clinical development plans and timelines, and the regulatory process for Athira product candidates; Athira may not be able to recruit sufficient patients for its clinical trials; future potential regulatory milestones of fosgonimeton and other product candidates, including those related to current and planned clinical studies may be insufficient to support regulatory submissions or approval; the outcome of legal proceedings which have been or may in the future be instituted against us and certain of our directors and officers; clinical trials may not demonstrate safety and efficacy of any of Athira’s product candidates; possible negative interactions of Athira's product candidates with other treatments; Athira’s assumptions regarding the sufficiency of its cash, cash equivalents and investments to fund its planned operations may be incorrect; while P300 latency is a functional measure that is highly correlated with cognition, Athira may not successfully establish a connection between these P300 latency results and improved cognition; adverse conditions in the general domestic and global economic markets; the impact of competition; regulatory agencies may be delayed in reviewing, commenting on or approving any of Athira’s clinical development plans as a result of the COVID-19 pandemic, which could further delay development timelines; the impact of expanded product development and clinical activities on operating expenses; the impact of new or changing laws and regulations; as well as the other risks detailed in Athira’s filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof and Athira undertakes no obligation to update forward-looking statements. Athira may not actually achieve the plans, intentions, or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the forward-looking statements.Investor & Media Contact:
Julie Rathbun
Julie.rathbun@athira.com
206-769-9219Graphics accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/f9fd4421-64ca-4764-bb84-a0bb9fc08a2d
https://www.globenewswire.com/NewsRoom/AttachmentNg/0bdf181e-7f88-4783-bcb5-7379e91819fd